Full-Services Capability
From small custom engineering solution to large scale production we have the resources to support your project regardless of scale by considereing your supply chanin to became your single source partner to allow us to be the one stop solution to all your project need satisfying unquie application and performance needs while maintaning tight repeatable out comes(Medical)
Our Approach
We utilize an Agile team approach for the development of products consisting of three development teams and one support team. All arunder the general direction of our customers and support of our regional team leaders. We utilize our IOS 13485 design controls and the designated teams to apply our broad experience in a controlled manner.
Design & Development
Freeway (Fuqing) medical offers the full range of design, prototyping, and development services. We turn your device concept into manufacturable design, creating a path to transfer it to initial production, and ultimately, to full-scale production.
Vertical lntegration is a core strength at ProXCath Medical . From concept to production, we canhandle:
- Equipment design
- Tool & fixture design & fabrication3D printing
- Braiding
- Coiling
- Vertical lamination
- Laser cutting & bonding
- Balloon forming & attachment
- Nitinol fabrication & heat setting
- RF weldingUltrasonic welding
- Swaging
- Plasma treatment
- Silicone LIM
- Molding
- Pad printing

Catheterinsnections s Testing
Catheter Testing
IS0 10555 catheter Testing
Intravascular catheters are designed for tasks such as supporting delivery ofmedicine and implantable devices into the human body. The best way toensure that these devices are safe, retain their quality, and performsuccessfully is to subject them to a series of rigorous performance anddurability tests.
Our catheter testing services are performed according to pre-approvedprotocols and conform with required lSO and ASTM standards. We can assistyou with your testing requirements and prepare your device for regulatorysubmission.
IS0 10555 Part 1,3, 4, 5, Intravascular Catheters -Sterile and Single-Use
IS0 10555 defines tests for intravascular sterile use catheters. Cathetertypes that can be tested according to this standard include: generalintravascular catheters, angiographic catheters, central venous cathetersballoon dilatation catheters and over-needle peripheral catheters
Device Prototyping
With hundreds of materials and tools in stock and countless configurations readily available, we can rapidly produce prototypes to accelerate your development projects.
Early prototypes are built in our laboratory which is stocked with tubing, adhesives, and mandrels, and equipped with a wide range of equipment, including:
- 3D printing
- Braiding
- Coiling
- Vertical lamination
- Laser cutting and bonding
- Balloon forming and
- Molding
- Pad printing
- attachment
- Nitinol fabrication and heat setting
- RF welding
- Ultrasonic welding
- Swaging
- Plasma treatment
- Silicone LIM
PRODUCT MENU
PERIPHERAL (PTA 018 )
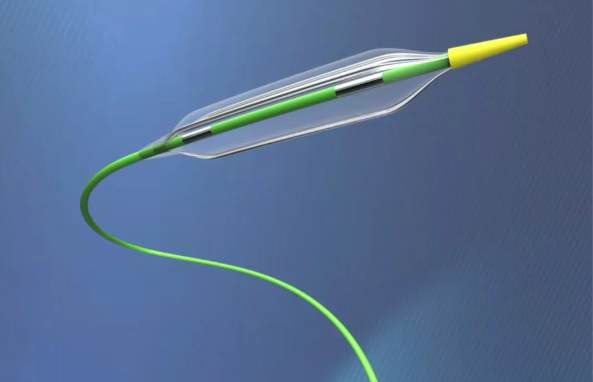
CORONARY(PTA OTW 035)
